Psoriasis
| Psoriasis | |
|---|---|
| Classification and external resources | |
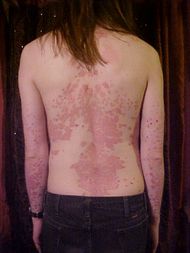 A person whose back and arms are affected by psoriasis |
|
| ICD-10 | L40. |
| ICD-9 | 696 |
| OMIM | 177900 |
| DiseasesDB | 10895 |
| MedlinePlus | 000434 |
| eMedicine | emerg/489 Dermatology:derm/365 plaque derm/361 guttate derm/363 nails derm/366 pustular Arthritis derm/918 Radiology radio/578 Physical Medicine pmr/120 |
| MeSH | D011565 |
Psoriasis (pronounced /səˈraɪ.əsɨs/) is a chronic, autoimmune disease that appears on the skin. It occurs when the immune system sends out faulty signals that speed up the growth cycle of skin cells. Psoriasis is not contagious.[1] It commonly causes red, scaly patches to appear on the skin, although some patients have no dermatological symptoms. The scaly patches commonly caused by psoriasis, called psoriatic plaques, are areas of inflammation and excessive skin production. Skin rapidly accumulates at these sites which gives it a silvery-white appearance. Plaques frequently occur on the skin of the elbows and knees, but can affect any area including the scalp, palms of hands and soles of feet, and genitals. In contrast to eczema, psoriasis is more likely to be found on the outer side of the joint.
The disorder is a chronic recurring condition that varies in severity from minor localized patches to complete body coverage. Fingernails and toenails are frequently affected (psoriatic nail dystrophy) and can be seen as an isolated symptom. Psoriasis can also cause inflammation of the joints, which is known as psoriatic arthritis. Ten to fifteen percent of people with psoriasis have psoriatic arthritis.[2],
The cause of psoriasis is not fully understood, but it is believed to have a genetic component and local psoriatic changes can be triggered by an injury to the skin known as Koebner phenomenon. Various environmental factors have been suggested as aggravating to psoriasis including stress, withdrawal of systemic corticosteroid, excessive alcohol consumption, and smoking but few have shown statistical significance.[3] There are many treatments available, but because of its chronic recurrent nature psoriasis is a challenge to treat.
Contents |
Classification
The symptoms of psoriasis can manifest in a variety of forms. Variants include plaque, pustular, guttate and flexural psoriasis. This section describes each type (with ICD-10 code [5]).[4]
Psoriasis is a chronic relapsing disease of the skin that may be classified into nonpustular and pustular types as follows[5]:414:
Nonpustular psoriasis
- Psoriasis vulgaris (Chronic stationary psoriasis, Plaque-like psoriasis). Plaque psoriasis (psoriasis vulgaris) (L40.0) is the most common form of psoriasis. It affects 80 to 90% of people with psoriasis. Plaque psoriasis typically appears as raised areas of inflamed skin covered with silvery white scaly skin. These areas are called plaques.
- Psoriatic erythroderma (Erythrodermic psoriasis). Erythrodermic psoriasis (L40.85) involves the widespread inflammation and exfoliation of the skin over most of the body surface. It may be accompanied by severe itching, swelling and pain. It is often the result of an exacerbation of unstable plaque psoriasis, particularly following the abrupt withdrawal of systemic treatment. This form of psoriasis can be fatal, as the extreme inflammation and exfoliation disrupt the body's ability to regulate temperature and for the skin to perform barrier functions.[6]
Pustular psoriasis
Pustular psoriasis (L40.1-3, L40.82) appears as raised bumps that are filled with non-infectious pus (pustules). The skin under and surrounding the pustules is red and tender. Pustular psoriasis can be localised, commonly to the hands and feet (palmoplantar pustulosis), or generalised with widespread patches occurring randomly on any part of the body.
- Generalized pustular psoriasis (Pustular psoriasis of von Zumbusch)
- Pustulosis palmaris et plantaris (Persistent palmoplantar pustulosis, Pustular psoriasis of the Barber type, Pustular psoriasis of the extremities)
- Annular pustular psoriasis
- Acrodermatitis continua
- Impetigo herpetiformis
Other psoriasis
Additional types of psoriasis include[7]:191-197:
- Drug-induced psoriasis
- Inverse psoriasis. Flexural psoriasis (inverse psoriasis) (L40.83-4) appears as smooth inflamed patches of skin. It occurs in skin folds, particularly around the genitals (between the thigh and groin), the armpits, under an overweight stomach (pannus), and under the breasts (inframammary fold). It is aggravated by friction and sweat, and is vulnerable to fungal infections.
- Napkin psoriasis
- Seborrheic-like psoriasis
Guttate psoriasis (L40.4) is characterized by numerous small, scaly, red or pink, teardrop-shaped lesions. These numerous spots of psoriasis appear over large areas of the body, primarily the trunk, but also the limbs, and scalp. Guttate psoriasis is often preceded by a streptococcal infection, typically streptococcal pharyngitis. The reverse is not true.
Nail psoriasis (L40.86) produces a variety of changes in the appearance of finger and toe nails. These changes include discolouring under the nail plate, pitting of the nails, lines going across the nails, thickening of the skin under the nail, and the loosening (onycholysis) and crumbling of the nail.
Psoriatic arthritis (L40.5) involves joint and connective tissue inflammation. Psoriatic arthritis can affect any joint but is most common in the joints of the fingers and toes. This can result in a sausage-shaped swelling of the fingers and toes known as dactylitis. Psoriatic arthritis can also affect the hips, knees and spine (spondylitis). About 10-15% of people who have psoriasis also have psoriatic arthritis.
Signs and symptoms
|
Plaque of psoriasis |
Plaque of psoriasis |
 An arm covered with plaque psoriasis |
 Psoriasis of a fingernail |
Quality of life
Psoriasis has been shown to affect health-related quality of life to an extent similar to the effects of other chronic diseases such as depression, myocardial infarction, hypertension, congestive heart failure or type 2 diabetes.[8] Depending on the severity and location of outbreaks, individuals may experience significant physical discomfort and some disability. Itching and pain can interfere with basic functions, such as self-care, walking, and sleep. Plaques on hands and feet can prevent individuals from working at certain occupations, playing some sports, and caring for family members or a home. Plaques on the scalp can be particularly embarrassing as flaky plaque in the hair can be mistaken for dandruff. Medical care can be costly and time-consuming and can interfere with an employment or school schedule.
Individuals with psoriasis may also feel self-conscious about their appearance and have a poor self-image that stems from fear of public rejection and psychosexual concerns. Psychological distress can lead to significant depression and social isolation.
In a 2008 National Psoriasis Foundation survey of 426 psoriasis sufferers, 71 percent reported that the disease was a significant problem in everyday life. More than half reported significant feelings of self-consciousness (63 percent) and embarrassment (58 percent). More than one-third said they avoided social activities and limited dating or intimate interactions.[9]
Many tools exist to measure quality of life of patients with psoriasis and other dermatalogical disorders. Clinical research has indicated that individuals often experience a diminished quality of life.[10] A 2009 study looked at the impact of psoriasis by using interviews with dermatologists and exploring patients viewpoint. It found that in cases of mild and severe psoriasis, itch contributed most to the diminished health-related quality of life (HRQoL).[11]
Severity

Psoriasis is usually graded as mild (affecting less than 3% of the body), moderate (affecting 3-10% of the body) or severe. Several scales exist for measuring the severity of psoriasis. The degree of severity is generally based on the following factors: the proportion of body surface area affected; disease activity (degree of plaque redness, thickness and scaling); response to previous therapies; and the impact of the disease on the person.
The Psoriasis Area Severity Index (PASI) is the most widely used measurement tool for psoriasis. PASI combines the assessment of the severity of lesions and the area affected into a single score in the range 0 (no disease) to 72 (maximal disease).[12] Nevertheless, the PASI can be too unwieldy to use outside of trials, which has led to attempts to simplify the index for clinical use.[13]
Cause
The cause of psoriasis is not fully understood. There are two main hypotheses about the process that occurs in the development of the disease. The first considers psoriasis as primarily a disorder of excessive growth and reproduction of skin cells. The problem is simply seen as a fault of the epidermis and its keratinocytes. The second hypothesis sees the disease as being an immune-mediated disorder in which the excessive reproduction of skin cells is secondary to factors produced by the immune system. T cells (which normally help protect the body against infection) become active, migrate to the dermis and trigger the release of cytokines (tumor necrosis factor-alpha TNFα, in particular) which cause inflammation and the rapid production of skin cells. It is not known what initiates the activation of the T cells.
The immune-mediated model of psoriasis has been supported by the observation that immunosuppressant medications can clear psoriasis plaques. However, the role of the immune system is not fully understood, and it has recently been reported that an animal model of psoriasis can be triggered in mice lacking T cells.[14] Animal models, however, reveal only a few aspects resembling human psoriasis.
Compromised skin barrier function has a role in psoriasis susceptibility.[15]
Psoriasis is a fairly idiosyncratic disease. The majority of people's experience of psoriasis is one in which it may worsen or improve for no apparent reason. Studies of the factors associated with psoriasis tend to be based on small (usually hospital based) samples of individuals. These studies tend to suffer from representative issues, and an inability to tease out causal associations in the face of other (possibly unknown) intervening factors. Conflicting findings are often reported. Nevertheless, the first outbreak is sometimes reported following stress (physical and mental), skin injury, and streptococcal infection. Conditions that have been reported as accompanying a worsening of the disease include infections, stress, and changes in season and climate. Certain medicines, including lithium salt, beta blockers and the Antimalarial drug chloroquininine have been reported to trigger or aggravate the disease. Excessive alcohol consumption, smoking and obesity may exacerbate psoriasis or make the management of the condition difficult or perhaps these comorbidities are effects rather than causes.[16][17] Hairspray, some face creams and hand lotions, can also cause an outbreak of psoriasis. In 1975, Stefania Jablonska and collaborators advanced a new theory that special antibodies tend to break through into the lower layers of the skin and set up a complex series of chemical reactions.[18]
Individuals suffering from the advanced effects of the Human immunodeficiency virus, or HIV, often exhibit psoriasis.[19] This presents a paradox to researchers as traditional therapies that reduce T-cell counts generally cause psoriasis to improve. Yet, as CD4-T-cell counts decrease with the progression of HIV, psoriasis worsens.[20] In addition, HIV is typically characterized by a strong Th2 cytokine profile, whereas psoriasis vulgaris is characterized by a strong Th1 secretion pattern.[21] It is hypothesized that the diminished CD4-T-Cell presence causes an over-activation of CD8-T-Cells, which are responsible for the exacerbation of psoriasis in HIV positive patients. It is important to remember that most individuals with psoriasis are otherwise healthy and the presence of HIV accounts for less than 1% of cases. The prevalence of psoriasis in the HIV positive population ranges from 1 to 6 percent, which is about 3 times higher than the normal population.[22] Psoriasis in AIDS sufferers is often severe, and untreatable with conventional therapy.[23]
Psoriasis occurs more likely in dry skin than oily or well-moisturized skin, and specifically after an external skin injury such as a scratch or cut (see Koebner phenomenon). This is believed to be caused by an infection, in which the infecting organism thrives under dry skin conditions with minimal skin oil, which otherwise protects skin from infections. The case for psoriasis is opposite to the case of athlete's foot, which occurs because of a fungus infection under wet conditions as opposed to dry in psoriasis. This infection induces inflammation, which causes the symptoms commonly associated with psoriasis, such as itching and rapid skin turnover, and leads to drier skin as the infecting organism absorbs the moisture that would otherwise go to the skin. To prevent dry skin and reduce psoriasis symptoms, it is advised to not use shower scrubs, as they not only damage skin by leaving tiny scratches, they also scrape off the naturally occurring skin oil. It is recommended to use talc powder after washing as that helps absorb excess moisture which would otherwise go to the infecting agent. Additionally, moisturizers can be applied to moisturize the skin, and lotions used to promote skin oil gland functions.
Genetic factors
Psoriasis has a large hereditary component, and many genes are associated with it, but it is not clear how those genes work together. Most of them involve the immune system, particularly the major histocompatibility complex (MHC) and T cells. The main value of genetic studies is that they identify molecular mechanisms and pathways for further study and potential drug targets.[24]
Classic genomewide linkage analysis has identified nine locations (loci) on different chromosomes that are associated with psoriasis. They are called psoriasis susceptibility 1 through 9 (PSORS1 through PSORS9). Within those loci are genes. Many of those genes are on pathways that lead to inflammation. Certain variations (mutations) of those genes are commonly found in psoriasis.[24]
The major determinant is PSORS1, which probably accounts for 35-50% of its heritability. It controls genes that affect the immune system or encode proteins that are found in the skin in greater amounts in psoriasis. PSORS1 is located on chromosome 6 in the MHC, which controls important immune functions. Three genes in the PSORS1 locus have a strong association with psoriasis vulgaris: HLA-C variant HLA-Cw6, which encodes a MHC class I protein; CCHCR1, variant WWC, which encodes a coiled protein that is overexpressed in psoriatic epidermis; and CDSM, variant allele 5, which encodes corneodesmosin, which is expressed in the granular and cornified layers of the epidermis and upregulated in psoriasis.[24]
Genomewide association scans have identified other genes which are altered to characteristic variants in psoriasis. Some of these genes express inflammatory signal proteins, which affect cells in the immune system that are also involved in psoriasis. Some of these genes are also involved in other autoimmune diseases.[24]
Two major genes under investigation are IL12B on chromosome 5q which expresses interleukin-12B; and IL23R on chromosome 1p which expresses the interleukin-23 receptor, and is involved in T cell differentiation. T cells are involved in the inflammatory process that leads to psoriasis.[24]
These genes are on the pathway that ends up upregulating tumor necrosis factor-α and nuclear factor κB, two genes that are involved in inflammation.[24]
Immunological factors
In psoriasis, immune cells move from the dermis to the epidermis, where they stimulate skin cells (keratinocytes) to proliferate. Psoriasis does not seem to be a true autoimmune disease.[24] In an autoimmune disease, the immune system confuses an outside antigen with a normal body component, and attacks them both. But in psoriasis, the inflammation doesn't seem to be caused by outside antigens (although DNA does have an immunostimulatory effect). Researchers have identified many of the immune cells that are involved in psoriasis, and the chemical signals they send to each other to coordinate inflammation. At the end of this process, immune cells such as dendritic cells and T cells move from the dermis to the epidermis, secreting chemical signals, such as tumor necrosis factor-α, interleukin-1β, and interleukin-6, which cause inflammation, and interleukin-22, which causes keratinocytes to proliferate.[24]
The immune system consists of an innate immune system, and an adaptive immune system.
In the innate system, immune cells have receptors that have evolved to target specific proteins and other antigens which are commonly found on pathogens. In the adaptive immune system, immune cells respond to proteins and other antigens that they may never have seen before, which are presented to them by other cells. The innate system often passes antigens on to the adaptive system. When the immune system makes a mistake, and identifies a healthy part of the body as a foreign antigen, the immune system attacks that protein, as it does in autoimmunity.
In psoriasis, DNA is an inflammatory stimulus. DNA stimulates the receptors on plasmacytoid dendritic cells, which produce interferon-α, an immune stimulatory signal (cytokine). In psoriasis, keratinocytes produce antimicrobial peptides. In response to dendritic cells and T cells, they also produce cytokines, such as interleukin-1, interleukin-6, and tumor necrosis factor-α, which signals more inflammatory cells to arrive and produces further inflammation.[24]
Dendritic cells bridge the innate and adaptive immune system. They are increased in psoriatic lesions and induce the proliferation of T cells and type 1 helper T cells. Certain dendritic cells can produce tumor necrosis factor-alpha, which calls more immune cells and stimulates more inflammation. Targeted immunotherapy, and psoralen and ultraviolet A (PUVA) therapy, reduces the number of dendritic cells.[24]
T cells move from the dermis into the epidermis. They are attracted to the epidermis by alpha-1 beta-1 integrin, a signalling molecule on the collagen in the epidermis. Psoriatic T cells secrete interferon-γ and interleukin-17. Interleukin-17 is also associated with interleukin-22. Interleukin-22 induces keratocytes to proliferate.[24]
One hypothesis is that psoriasis involves a defect in regulatory T cells, and in the regulatory cytokine interleukin-10.[24]
Diagnosis
A diagnosis of psoriasis is usually based on the appearance of the skin. There are no special blood tests or diagnostic procedures for psoriasis. Sometimes a skin biopsy, or scraping, may be needed to rule out other disorders and to confirm the diagnosis. Skin from a biopsy will show clubbed Rete pegs if positive for psoriasis. Another sign of psoriasis is that when the plaques are scraped, one can see pinpoint bleeding from the skin below (Auspitz's sign).
Management

Research in the past decade has led to "new, highly effective targeted therapies," with phase III data or regulatory approval. They make use of research into how immune cells like T cells and dendrocytes travel, and how they use chemical signals (cytokines) to interact with each other. The drugs follow two strategies: anti-T cell strategies and anticytokine strategies.[24]
Two drugs that target T cells are efalizumab and alefacept. Efalizumab (which is no longer marketed) is a monoclonal antibody which blocks the molecules that dendritic cells use to communicate with T cells. It also blocks the adhesion molecules on the endothelial cells that line blood vessels, which attract T cells. However, it suppressed the immune system's ability to control normally harmless viruses, which led to fatal brain infections. Alefacept also blocks the molecules that dendritic cells use to communicate with T cells, and even causes natural killer cells to kill T cells, as a way of controlling inflammation.[24]
Several monoclonal antibodies (MABs) target cytokines, the molecules that cells use to send inflammatory signals to each other. One of the main inflammatory signals in the body is tumor necrosis factor α (TNF-α), and three MABs -- infliximab, adalimumab and etanercept bind to TNF-α. Two more inflammatory signals are interleukin-23 and interleukin-12. A protein chain, p40, is the same on both of those interleukins, and the monoclonal antibody ustekinumab binds to that common protein to interfere with both of them.[24]
There can be substantial variation between individuals in the effectiveness of specific psoriasis treatments. Because of this, dermatologists often use a trial-and-error approach to finding the most appropriate treatment for their patient. The decision to employ a particular treatment is based on the type of psoriasis, its location, extent and severity. The patient’s age, sex, quality of life, comorbidities, and attitude toward risks associated with the treatment are also taken into consideration.
In 2008, the FDA approved three new treatment options[25] available to psoriasis patients: 1) Taclonex Scalp, a new topical ointment for treating scalp psoriasis; 2) the Xtrac Velocity excimer laser system, which emits a high-intensity beam of ultraviolet light, can treat moderate to severe psoriasis; and 3) the biologic drug adalimumab (brand name Humira) was also approved to treat moderate to severe psoriasis. Adalimumab had already been approved to treat psoriatic arthritis.
Medications with the least potential for adverse reactions are preferentially employed. If the treatment goal is not achieved then therapies with greater potential toxicity may be used. Medications with significant toxicity are reserved for severe unresponsive psoriasis. This is called the psoriasis treatment ladder.[26] As a first step, medicated ointments or creams, called topical treatments, are applied to the skin. These treatments include non-steroidal creams such as Zithranol-RR, a topical cream containing anthralin. If topical treatment fails to achieve the desired goal then the next step would be to expose the skin to ultraviolet (UV) radiation. This type of treatment is called phototherapy. The third step involves the use of medications which are taken internally by pill or injection. This approach is called systemic treatment.
A 2010 meta-analysis compares the change in Psoriasis Area and Severity Index (PASI) improvement from baseline in 22 trials. The combination therapy for moderate to severe psoriasis using psoralen with ultraviolet A (PUVA) plus acitretin shows a 97.3% PASI improvement from baseline. Therapy limitations need to be taken into consideration in the treatment of moderate to severe psoriasis, such as the increased risk of skin cancer with phototherapy and birth defects with acitretin.[27]
Over time, psoriasis can become resistant to a specific therapy. Treatments may be periodically changed to prevent resistance developing (tachyphylaxis) and to reduce the chance of adverse reactions occurring. This is called treatment rotation.
Antibiotics are generally not indicated in routine treatment of psoriasis. However, antibiotics may be employed when an infection, such as that caused by the bacteria Streptococcus, triggers an outbreak of psoriasis, as in certain cases of guttate psoriasis.
Cognitive behaviour therapy
A psychological symptom management programme has been reported as being a helpful adjunct to traditional therapies in the management of psoriasis.[28]. In the UK The Psoriasis and Psoriatic Arthritis Alliance (PAPAA) a not-for-profit charity has funded research carried out by the University of Manchester, to develop a symptom management programme called Electronic Targeted Intervention for Psoriasis (eTIPs) using a modified Cognitive Behaviour Therapy model. This research follows research by Fortune D G et al.[29] on psychological stress, distress and disability in patients with psoriasis.
Topical treatment
Bath solutions and moisturizers, mineral oil, and petroleum jelly may help soothe affected skin and reduce the dryness which accompanies the build-up of skin on psoriatic plaques. Medicated creams and ointments applied directly to psoriatic plaques can help reduce inflammation, remove built-up scale, reduce skin turn over, and clear affected skin of plaques. Ointment and creams containing coal tar, dithranol (anthralin), corticosteroids like desoximetasone (Topicort), fluocinonide, vitamin D3 analogues (for example, calcipotriol), and retinoids are routinely used. Argan oil has also been used with some promising results.[30] The use of the Finger tip unit may be helpful in guiding how much topical treatment to use.[31] The mechanism of action of each is probably different but they all help to normalise skin cell production and reduce inflammation. Activated vitamin D and its analogues are highly effective inhibitors of skin cell proliferation.
The disadvantages of topical agents are variably that they can often irritate normal skin, can be time consuming and awkward to apply, cannot be used for long periods, can stain clothing or have a strong odour. As a result, it is sometimes difficult for people to maintain the regular application of these medications. Abrupt withdrawal of some topical agents, particularly corticosteroids, can cause an aggressive recurrence of the condition. This is known as a rebound of the condition.
Some topical agents are used in conjunction with other therapies, especially phototherapy.
Phototherapy
It has long been recognized that daily, short, non-burning exposure to sunlight helped to clear or improve psoriasis in some patients. Niels Finsen was the first physician to investigate the therapeutic effects of sunlight scientifically and to use selected portions of the solar spectrum in clinical practice. This became known as phototherapy.
Sunlight contains many different wavelengths of light. It was during the early part of the 20th century that it was recognised that for psoriasis the therapeutic property of sunlight was due to the wavelengths classified as ultraviolet (UV) light.
Ultraviolet wavelengths are subdivided into UVA (380–315 nm) UVB (315–280 nm), and UVC (< 280 nm). Ultraviolet B (UVB) (315–280 nm) is absorbed by the epidermis and has a beneficial effect on psoriasis. There are two types of UVB lamps: Narrowband UVB (311 to 312 nm), and Broadband (Wideband, or "FS" type) UVB (290-320 nm). UVB Broadband is more erythemal and therefore requires shorter exposure time, while UVB Narrowband does not include the spectrum of less than 300 nanometers, allowing much higher doses without erythema, and thus considered safer. The UVB Narrowband lamp was developed by Philips Lighting specifically to match the action spectrum of psoriasis, with a sharp emission peak at 311 nm, to have increased effectiveness compared to broadband lamps.[32] Exposure to UVB several times per week, over several weeks can help people attain a remission from psoriasis. Sometimes it is needed to continue the treatments once a week as maintenance, or the chronic disease will return.
In hospitals, ultraviolet light treatment is frequently combined with topical (coal tar, calcipotriol) or systemic treatment (Retinoids) as there is a synergy in their combination. The Ingram regime involves UVB and the application of anthralin paste. The Goeckerman regime combines coal tar ointment with UVB. Because coal tar includes unknown ingredients that might cause cancer, and is a time intensive treatment, the use of coal tar has fallen out of favor.
Photochemotherapy
Psoralen and ultraviolet A phototherapy (PUVA) combines the oral or topical administration of psoralen with exposure to ultraviolet A (UVA) light. Precisely how PUVA works is not known. The mechanism of action probably involves activation of psoralen by UVA light which inhibits the abnormally rapid production of the cells in psoriatic skin. There are multiple mechanisms of action associated with PUVA, including effects on the skin immune system.
PUVA is associated with nausea, headache, fatigue, burning, and itching. Long-term treatment is associated with squamous cell carcinoma (not with melanoma).
Systemic treatment

Psoriasis that is resistant to topical treatment and phototherapy is treated by medications that are taken internally by pill or injection. This is called systemic treatment. Patients undergoing systemic treatment are required to have regular blood and liver function tests because of the toxicity of the medication. Pregnancy must be avoided for the majority of these treatments. Most people experience a recurrence of psoriasis after systemic treatment is discontinued.
The three main traditional systemic treatments are methotrexate, cyclosporine and retinoids. Methotrexate and cyclosporine are immunosuppressant drugs; retinoids are synthetic forms of vitamin A.
Other additional drugs, not specifically licensed for psoriasis, have been found to be effective. These include the antimetabolites tioguanine, mercaptopurine and fluorouracil, the cytotoxic agents hydroxyurea and paclitaxel, alkylating agents chlorambucil and cyclophosphamide, some DMARDs like sulfasalazine, colchicine, dapsone, the immunosuppressants mycophenolate mofetil, azathioprine and oral tacrolimus. These have all been used effectively to treat psoriasis when other treatments have failed. Although not licensed in many other countries, fumaric acid esters have also been used to treat severe psoriasis in Germany for over 20 years. There is also some evidence for beneficial effect on psoriasis of insulin-sensitizing drugs (thiazolidinediones like pioglitazone and rosiglitazone, and a more modest effect is described for metformin), somatostatin, bromocriptine, and some lipid-lowering drugs from the group of statines (like simvastatin), and omega-3 fatty acid supplements. For all those drugs it is hypothesised that their antipsoriatic activity comes from their immunomodulatory properties.
There are also case reports and small trials describing beneficial effects of yohimbine (effect is thought to be secondary to its insulin-lowering and growth hormone lowering properties), ketotifen (effect is thought to be secondary to its ability to dampen release of inflammatory mediators) and albuterol (beta-adrenergic agonist).
Antihistamine drugs generally do not help to improve psoriasis lesions, but they may be of use to reduce itching and also are helpful in cases where psoriasis coexists with skin allergy, for example chronic urticaria. Some antihistamines have sedative properties, thus might aid to improve sleep and reduce anxiety in psoriasis patients. Antidepressant medications may help to reduce comorbid depression, anxiety, social isolation, improve sleep and in some cases reduce itching (primarily due to antihistamine effects of tricyclic antidepressants and some SSRIs). Naltrexone, an opioid antagonist, and pregabalin or gabapentin, benzodiazepine anxiolytics are also of use in severe itching.
NSAID drugs generally do not help to improve psoriatic arthritis itself, but they might provide rapid symptomatic relief from pain and swelling.
Biologics are manufactured proteins that interrupt the immune process involved in psoriasis. Unlike generalised immunosuppressant therapies such as methotrexate, biologics focus on specific aspects of the immune function leading to psoriasis. These drugs (interleukin antagonists) are relatively new, and their long-term impact on immune function is unknown, but they have proven effective in treating psoriasis and psoriatic arthritis. They include Amevive, Enbrel, Humira, Remicade and Raptiva. Raptiva was withdrawn by its maker from the US market in April, 2009. Biologics are usually given by self-injection or in a doctor's office. They are very expensive and only suitable for very few patients with severe psoriasis. Ustekinumab (IL-12 and IL-23 blocker) shows hopeful results for psoriasis therapy.
In the United Kingdom in 2005 the British Association of Dermatologists (BAD) published guidelines for use of biological interventions in psoriasis.[33] A UK national register called the BAD Biological Register (BADBIR) has been set up to collect valuable information on side effects and benefits and will be used to inform doctors on how best to use biological agents and similar drugs.
Noting that botulinum toxin has been shown to have an effect on inhibiting neurogenic inflammation, and evidence suggesting the role of neurogenic inflammation in the pathogenesis of psoriasis, the University of Minnesota has begun a clinical trial to follow up on the observation that patients treated with botulinum toxin for dystonia had dramatic improvement in psoriasis. See: Use of Botulinum Toxin to Treat Psoriasis.
Alternative therapy
Some studies suggest that psoriasis symptoms can be relieved by changes in diet and lifestyle. Fasting periods, low energy diets and vegetarian diets have improved psoriasis symptoms in some studies, and diets rich in fatty acids from fish oil have also shown beneficial effects.[34] The severity of psoriasis symptoms may also be influenced by lifestyle habits related to alcohol, smoking, weight, sleep, stress and exercise.[35]
Climatotherapy involves the notion that some diseases can be successfully treated by living in a particular climate. Several psoriasis clinics are located throughout the world based on this idea. The Dead Sea is one of the most popular locations for this type of treatment.
Another treatment is ichthyotherapy, which is practised at some spas in Turkey, Croatia, Ireland, Hungary and Serbia. In this therapy, doctor fish are encouraged to feed on the psoriatic skin of people with psoriasis. The fish, which live in outdoor pools, only consume the affected areas of the skin. The outdoor location of the spa may also have a beneficial effect. This treatment can provide temporary relief of symptoms. A revisit to the spas every few months is often required. Treatment in this hot spring has been examined in two small clinical trials, with positive results.[36][37]
Oregon-grape (Mahonia Aquifolium) is said to be effective in the treatment of eczema and psoriasis.[38][39][40]
Prognosis
Psoriasis is a lifelong condition.[41] There is currently no cure but various treatments can help to control the symptoms. Many of the most effective agents used to treat severe psoriasis carry an increased risk of significant morbidity including skin cancers, lymphoma and liver disease. However, the majority of people's experience of psoriasis is that of minor localized patches, particularly on the elbows and knees, which can be treated with topical medication. Psoriasis can get worse over time but it is not possible to predict who will go on to develop extensive psoriasis or those in whom the disease may appear to vanish. Individuals will often experience flares and remissions throughout their lives. Controlling the signs and symptoms typically requires lifelong therapy.
According to one study,[42] psoriasis is linked to 2.5-fold increased risk for non melanoma skin cancer in men and women, with no preponderance of any specific histologic subtype of cancer. This increased risk could also be attributed to antipsoriatic treatment.
Epidemiology
Psoriasis affects both sexes equally and can occur at any age, although it most commonly appears for the first time between the ages of 15 and 25 years.
The prevalence of psoriasis in Western populations is estimated to be around 2-3%. The prevalence of psoriasis among 7.5 million patients who were registered with a general practitioner in the United Kingdom was 1.5%.[43] A survey[44] conducted by the National Psoriasis Foundation (a US based psoriasis education and advocacy group) found a prevalence of 2.1% among adult Americans. The study found that 35% of people with psoriasis could be classified as having moderate to severe psoriasis.
Around one-third of people with psoriasis report a family history of the disease, and researchers have identified genetic loci associated with the condition. Studies of monozygotic twins suggest a 70% chance of a twin developing psoriasis if the other twin has psoriasis. The concordance is around 20% for dizygotic twins. These findings suggest both a genetic predisposition and an environmental response in developing psoriasis.[45]
Onset before age 40 usually indicates a greater genetic susceptibility and a more severe or recurrent course of psoriasis.
History

Psoriasis is probably one of the longest known illnesses of humans and simultaneously one of the most misunderstood. Some scholars believe psoriasis to have been included among the skin conditions called tzaraat in the Bible.[46] In more recent times psoriasis was frequently described as a variety of leprosy. The Greeks used the term lepra (λεπρα) for scaly skin conditions. They used the term psora to describe itchy skin conditions. It became known as Willan's lepra in the late 18th century when English dermatologists Robert Willan and Thomas Bateman differentiated it from other skin diseases. Leprosy, they said, is distinguished by the regular, circular form of patches, while psoriasis is always irregular. Willan identified two categories: leprosa graecorum and psora leprosa.[47]
While it may have been visually, and later semantically, confused with leprosy, it was not until 1841 that the condition was finally given the name psoriasis by the Viennese dermatologist Ferdinand von Hebra. The name is derived from the Greek word psora which means to itch.[48]
It was during the 20th century that psoriasis was further differentiated into specific types.
Historical treatment
The history of psoriasis is littered with treatments of dubious effectiveness and high toxicity. These treatments received brief popularity at particular time periods or within certain geographical regions. The application of cat faeces to red lesions on the skin, for example, was one of the earliest topical treatments employed in ancient Egypt. Onions, sea salt and urine, goose oil and semen, wasp droppings in sycamore milk, and soup made from vipers have all been reported as being ancient treatments.
In the more recent past Fowler's solution, which contains a poisonous and carcinogenic arsenic compound, was used by dermatologists as a treatment for psoriasis during the 18th and 19th centuries. Grenz rays (also called ultrasoft X-rays or Bucky rays) was a popular treatment of psoriasis during the middle of the 20th century. This type of therapy was superseded by ultraviolet therapy.
Undecylenic acid was investigated and used for psoriasis some 40 years ago(cir. 1950~).[49]
All these treatments have fallen out of favour.
Sulphur was fashionable as a treatment for psoriasis in the Victorian and Edwardian eras. It has recently re-gained some credibility as a safe alternative to steroids and coal tar.
Research
Historically, agents used to treat psoriasis were discovered by experimentation or by accident. In contrast, current novel therapeutic agents are designed from a better understanding of the immune processes involved in psoriasis and by the specific targeting of molecular mediators. Examples can be seen in the use of biologics which target T cells and TNF inhibitors.
It has been suggested that cannabis might treat psoriasis, due to the anti-inflammatory properties of its cannabinoids, and the regulatory effects of THC on the immune system.[50] The adverse effects of cannabis might be overcome by use of more specific cannabinoid receptor medications,[51] to inhibit keratinocyte proliferation.[52]
Future innovation should see the creation of additional drugs that refine the targeting of immune-mediators further.[53]
Research into antisense oligonucleotides carries the potential to provide novel therapeutic strategies for treating psoriasis.[54]
ABT-874 is a human anti-IL-12 monoclonal antibody being developed by Abbott Laboratories in conjunction with Cambridge Antibody Technology for the treatment of multiple autoimmune diseases including psoriasis. Phase II trials have been completed and showed promising results.[55] Abbott was planning to initiate Phase III trials in 2007.[56]
In 2004, Tas and Avci [57] demonstrated cyclopamine’s clinical potential for the treatment of psoriasis and basal cell carcinoma in two preliminary proof of concept studies. By treating 31 psoriatic lesions in 7 patients, these authors asserted that topical cyclopamine was more effective in the clinical and histological clearance of guttate and plaque psoriasis than the topical steroid clobetasol-17 propionate. Furthermore, they demonstrated that concurrent application of cylopamine and clobetasol-17 propionate accelerated regression and clearance of selected lesions greater than cyclopamine alone with clearance times as early as 48 hours.They assert that cyclopamine inhibits the abnormal proliferation of epithelial cells, induces terminal differentiation, and is associated with the decreased presence of inflammatory cells, including CD41 lymphocytes.
On August 27, 2006, scientists led by Jeung-Hoon Lee created in the laboratory synthetic lipids called pseudoceramides which are involved in skin cell growth and could be used in treating skin diseases such as atopic dermatitis, a form of eczema characterized by red, flaky and very itchy skin; psoriasis, and glucocorticoid-induced epidermal atrophy, in which the skin shrinks due to skin cell loss.[58]
On November 17, 2008, scientists led by Yin-Ku Lin of Chang Gung Memorial Hospital and Chang Gung University in Taoyuan, Taiwan, told Reuters by telephone that Indigo naturalis (Qing Dai, 青黛), a dark blue plant used in traditional Chinese medicine, appears to be effective in treating psoriasis. In the latest issue of Archives of Dermatology, they wrote, "The indigo naturalis ointment-treated lesions showed an 81 percent improvement, the (non-medicated) ointment-treated lesions showed a 26 percent improvement."[59]
Talarozole amplifies the effects of retinoic acid by inhibiting its metabolism. As of February 2009[update], it is undergoing clinical trials.[60]
Psoriasis in children
Psoriasis can affect children. Approximately one third of psoriasis patients report being diagnosed before age 20.[61] Self-esteem and behavior can be affected by the disease. Bullying has been noted in clinical research.[62]
See also
- List of cutaneous conditions
- Psoriatic nails
References
- ↑ http://www.psoriasis.org/netcommunity/about_psoriasis
- ↑ http://www.ema.europa.eu/pdfs/human/ewp/245402en.pdf
- ↑ http://www.emedicine.com/derm/topic365.htm#section~clinical see causes
- ↑ "Application to dermatology of International Classification of Disease (ICD-10) - ICD sorted by code: L40.000 - L41.000", The International League of Dermatological Societies
- ↑ Freedberg, et al. (2003). Fitzpatrick's Dermatology in General Medicine. (6th ed.). McGraw-Hill. ISBN 0071380760.
- ↑ "Erythrodermic psoriasis", New Zealand Dermatological Society
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. ISBN 0721629210.
- ↑ Sampogna, F.: Chren, M.M.; Melchi, C.F.; Pasquin, P.; Tabolli, S.; Abeni, D. "Age, Gender, quality of life and psychological distress in patients hospitalized with psoriasis. British journal of dermatology: Feb2006, Vol. 154 Issue 2 pp 325-331
- ↑ "Coping With Psoriasis" Parade.com
- ↑ Bhosle, Monali J. et al. Quality of life in patients with psoriasis. Health and Quality of live outcomes. 2066 4:35. Accessed online 13 June 2009.
- ↑ Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 6 Jul 2009;7(1):62. PMID 19580674
- ↑ "Psoriasis Update -Skin & Aging". http://www.skinandaging.com/article/5394. Retrieved 2007-07-28.
- ↑ Louden BA, Pearce DJ, Lang W, Feldman SR (2004). "A Simplified Psoriasis Area Severity Index (SPASI) for rating psoriasis severity in clinic patients". Dermatol. Online J. 10 (2): 7. PMID 15530297.
- ↑ Zenz R, Eferl R, Kenner L, et al. (2005). "Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins". Nature 437 (7057): 369–75. doi:10.1038/nature03963. PMID 16163348.
- ↑ PMID 19169253
- ↑ [1] Psoriasis Triggers at Psoriasis Net. SkinCarePhysicians.com 9-28-05. American Academy of Dermatology, 2008.
- ↑ Behnam SM, Behnam SE, Koo JY (2005). "Smoking and psoriasis". Skinmed 4 (3): 174–6. doi:10.1111/j.1540-9740.2005.03716.x. PMID 15891254. http://www.lejacq.com/articleDetail.cfm?pid=SKINmed_4;3:174.
- ↑ Coleman, Lester L (November 30, 1975). "Your Health". Boca Raton News. http://news.google.com/newspapers?id=d9APAAAAIBAJ&sjid=WI0DAAAAIBAJ&pg=4808,3654533&dq.
- ↑ [2][3] Fife, Jeffes, Koo, Waller. Unraveling the Paradoxes of HIV-associated Psoriasis: A Review of T-cell Subsets and Cytokine Profiles. 5-18-07. Retrieved 5-13-08.
- ↑ Ortonne JP, Lebwohl M, Em Griffiths C (2003). "Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis". Eur J Dermatol 13 (2): 117–23. PMID 12695125. http://www.john-libbey-eurotext.fr/medline.md?issn=1167-1122&vol=13&iss=2&page=117.
- ↑ Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG (November 1999). "The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients". J. Invest. Dermatol. 113 (5): 752–9. doi:10.1046/j.1523-1747.1999.00749.x. PMID 10571730.
- ↑ [4] A Case Report of Severe Psoriasis in a Patient with AIDS: The Role of the HIV Virus and the Therapeutic Challenges Involved. Vol: 13 No 2, 2002. National Skin Center. Retrieved 05-13-08.
- ↑ http://cnx.org/content/m14956/latest/
- ↑ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 24.10 24.11 24.12 24.13 24.14 Review article: Mechanisms of Disease. Psoriasis. Frank O. Nestle, Daniel H. Kaplan, and Jonathan Barker. N Engl J Med, 361:496-509, July 30, 2009
- ↑ "Psoriasis Medical Breakthroughs" Parade.com
- ↑ Lofholm PW (2000). "The psoriasis treatment ladder: a clinical overview for pharmacists". US Pharm 25 (5): 26–47. http://www.uspharmacist.com/oldformat.asp?url=newlook/files/Feat/apr00pro.cfm&pub_id=8&article_id=511.
- ↑ Hankin CS, Bhatia ND, Goldenberg G, et al. A Comparison of the Clinical Effectiveness and Cost-Effectiveness of Treatments for Moderate to Severe Psoriasis. Drug Benefit Trends. 2010;21:17-27.
- ↑ "Research Findings Register: summary number 637". http://www.refer.nhs.uk/ViewRecord.asp?ID=637&Print=1. Retrieved 2007-07-22.
- ↑ Fortune, D.G., Richards, H.L., Griffiths, C.E.M., & Main, C.J.(2002). Psychological stress, distress and disability in patients with psoriasis: Consensus and variation in the contribution of illness perceptions, coping and alexithymia. British Journal of Clinical Psychology, 41, 157-174.
- ↑ "Irishman hits on 'cure' for psoriasis". Belfast Telegraph. December 12, 2007. http://www.belfasttelegraph.co.uk/news/local-national/article3247531.ece. Retrieved 2007-12-13.
- ↑ Menter A, Korman NJ, Elmets CA et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. J Am Acad Dermatol 2009; 60: 643-659.
- ↑ Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris, Walters, et al. Journal of the American Academy of Dermatology, Volume 40, Issue 6, June 1999, Pages 893-900
- ↑ British Association of Dermatologists guidelines for use of biological interventions in psoriasis 2005 (C H Smith, A V Anstey, J N Barker, A D Burden, R J Chalmers, D Chandler, A Y Finlay, C E Griffiths, K Jackson, N J McHugh, K E McKenna, N J Reynolds , A D Ormerod). BJD, Vol. 153, No. 3, September 2005 (p486)
- ↑ Wolters M (October 2005). "Diet and psoriasis: experimental data and clinical evidence". British Journal of Dermatology 153 (4): 706–714. doi:10.1111/j.1365-2133.2005.06781.x. PMID 16181450. http://www.ncbi.nlm.nih.gov/pubmed/16181450.
- ↑ Treloar V (2010). "Integrative dermatology for psoriasis: facts and controversies". Clinics in Dermatology 28 (1): 93–99. doi:10.1016/j.clindermatol.2009.03.016. PMID 20082958. http://www.ncbi.nlm.nih.gov/pubmed/20082958.
- ↑ Ozçelik S, Polat HH, Akyol M, Yalçin AN, Ozçelik D, Marufihah M (June 2000). "Kangal hot spring with fish and psoriasis treatment". J. Dermatol. 27 (6): 386–90. PMID 10920584.
- ↑ Grassberger M, Hoch W (December 2006). "Ichthyotherapy as alternative treatment for patients with psoriasis: a pilot study". Evid Based Complement Alternat Med 3 (4): 483–8. doi:10.1093/ecam/nel033. PMID 17173112.
- ↑ Donsky, Howard; Don Clarke. "Relieva, a Mahonia Aquifolium Extract for the Treatment of Adult Patients With Atopic Dermatitis". http://www.americantherapeutics.com/pt/re/ajt/abstract.00045391-200709000-00008.htm;jsessionid=HnrPMH6R3JhTFQNQphZtJqdp7608hvDvWLt5sm4Wj7pW52SdRL4W!1821113646!181195629!8091!-1. Retrieved 4 November 2007.
- ↑ Rackova L, Oblozinsky M, Kostalova D, Kettmann V, Bezakova L (2007). "Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids". J Inflamm (Lond) 4: 15. doi:10.1186/1476-9255-4-15. PMID 17634120.
- ↑ Bernstein, Steve et al.. "Treatment of Mild to Moderate Psoriasis with Relieva, a Mahonia aquifolium Extract-A Double-Blind, Placebo-Controlled Study". http://www.americantherapeutics.com/pt/re/ajt/abstract.00045391-200603000-00007.htm;jsessionid=Hnyhk0cdJylLgL8fyNyYdpmy1WpycJvHGpLKFWpfFhX849clNk8j!1821113646!181195629!8091!-1. Retrieved 4 November 2007.
- ↑ Jobling R (2007). "A patient's journey:Psoriasis". Br Med J 334 (7600): 953–4. doi:10.1136/bmj.39184.615150.802. PMID 17478850.
- ↑ Olsen JH, Frentz G, Møller H (1993). "[Psoriasis and cancer]" (in Danish). Ugeskr. Laeg. 155 (35): 2687–91. PMID 8212383.
- ↑ Gelfand JM et al. (2005). "Prevalence and Treatment of Psoriasis in the United Kingdom". Arch. Dermatol. 141 (12): 1537–1541. doi:10.1001/archderm.141.12.1537. PMID 16365254.
- ↑ "BENCHMARK SURVEY ON PSORIASIS AND PSORIATIC ARTHRITIS: SUMMARY OF TOP-LINE RESULTS" (PDF). NATIONAL PSORIASIS FOUNDATION. http://www.psoriasis.org/files/pdfs/press/npfsurvey.pdf.
- ↑ Krueger G, Ellis CN (2005). "Psoriasis--recent advances in understanding its pathogenesis and treatment". J. Am. Acad. Dermatol. 53 (1 Suppl 1): S94–100. doi:10.1016/j.jaad.2005.04.035. PMID 15968269.
- ↑ Shai A, Vardy D, Zvulunov A (2002). "[Psoriasis, biblical afflictions and patients' dignity]" (in Hebrew). Harefuah 141 (5): 479–82, 496. PMID 12073533.
- ↑ A note on the history of psoriasis. Irish Journal of Medical Science.Volume 30, Number 3. March 1955 pp 141-142
- ↑ Glickman FS (1986). "Lepra, psora, psoriasis". J. Am. Acad. Dermatol. 14 (5 Pt 1): 863–6. doi:10.1016/S0190-9622(86)70101-1. PMID 3519699.
- ↑ Ereaux L, Craig G (October 1949). "The Oral Administration Of Undecylenic Acid In The Treatment Of Psoriasis" (PDF). Canad. M. A. J. 61: 361–4. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1591667&blobtype=pdf. Retrieved 2007-01-05. - see page 4/364 of link
- ↑ Namazi MR (2005). "Cannabinoids, loratadine and allopurinol as novel additions to the antipsoriatic ammunition". Journal of the European Academy of Dermatology and Venereology : JEADV 19 (3): 319–22. doi:10.1111/j.1468-3083.2004.01184.x. PMID 15857457.
- ↑ Fowler CJ (2005). "Pharmacological properties and therapeutic possibilities for drugs acting upon endocannabinoid receptors". Current drug targets. CNS and neurological disorders 4 (6): 685–96. doi:10.2174/156800705774933041. PMID 16375686.
- ↑ Wilkinson JD, Williamson EM (2007). "Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis". J. Dermatol. Sci. 45 (2): 87–92. doi:10.1016/j.jdermsci.2006.10.009. PMID 17157480.
- ↑ Nickoloff BJ, Nestle FO (2004). "Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities". J. Clin. Invest. 113 (12): 1664–75. doi:10.1172/JCI200422147. PMID 15199399.
- ↑ White PJ, Atley LM, Wraight CJ (2004). "Antisense oligonucleotide treatments for psoriasis". Expert opinion on biological therapy 4 (1): 75–81. doi:10.1517/14712598.4.1.75. PMID 14680470.
- ↑ Heller M. (2007) Positive results for ABT-874 in the treatment of psoriasis J Drugs Dermatol
- ↑ Cambridge Antibody Technology | ABT-874
- ↑ [Tas S, Avci O. Rapid clearance of psoriatic skin lesions induced by topical cyclopamine. Dermatology 2004;209:126-31]
- ↑ Science Daily, New Skin-healing Chemicals
- ↑ Indigo plant may treat chronic skin disease (Reuters)
- ↑ Giltaire, S; Herphelin F, Frankart A, Hérin M, Stoppie P, Poumay Y (11 December 2008). "The CYP26 inhibitor R115866 potentiates the effects of all-trans retinoic acid on cultured human epidermal keratinocytes". Br J Dermatol 160 (3): 505–13. doi:10.1111/j.1365-2133.2008.08960.x. PMID 19120344.
- ↑ Benoit, Sandrine MD and Hamm, Henning MD. Childhood Psoriasis. Clinics in Dermatology: Volume 25, Issue 6 Nov-Dec 2007, pp 555-562. Abstract Accessed online 13 June 2009.
- ↑ Magin, Parker; Adams, Jon; Heading, Gaynor; Pond, Dimity; and Smith, Wayne. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results in a qualitative study. Scandanavian Journal of Caring Sciences; 2008; 22; 430-436.
External links
- "Questions and Answers about Psoriasis" at National Institute of Arthritis and Musculoskeletal and Skin Diseases
- Psoriasis at National Institute of Arthritis and Musculoskeletal and Skin Diseases
- National Psoriasis Foundation Homepage
- The Psoriasis Association
- The Mayo Clinic
- DermAtlas 7
Books
- From Arsenic to Biologicals: A 200 Year History of Psoriasis (Barbara S. Baker), ISBN 0-955-16032-4.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||